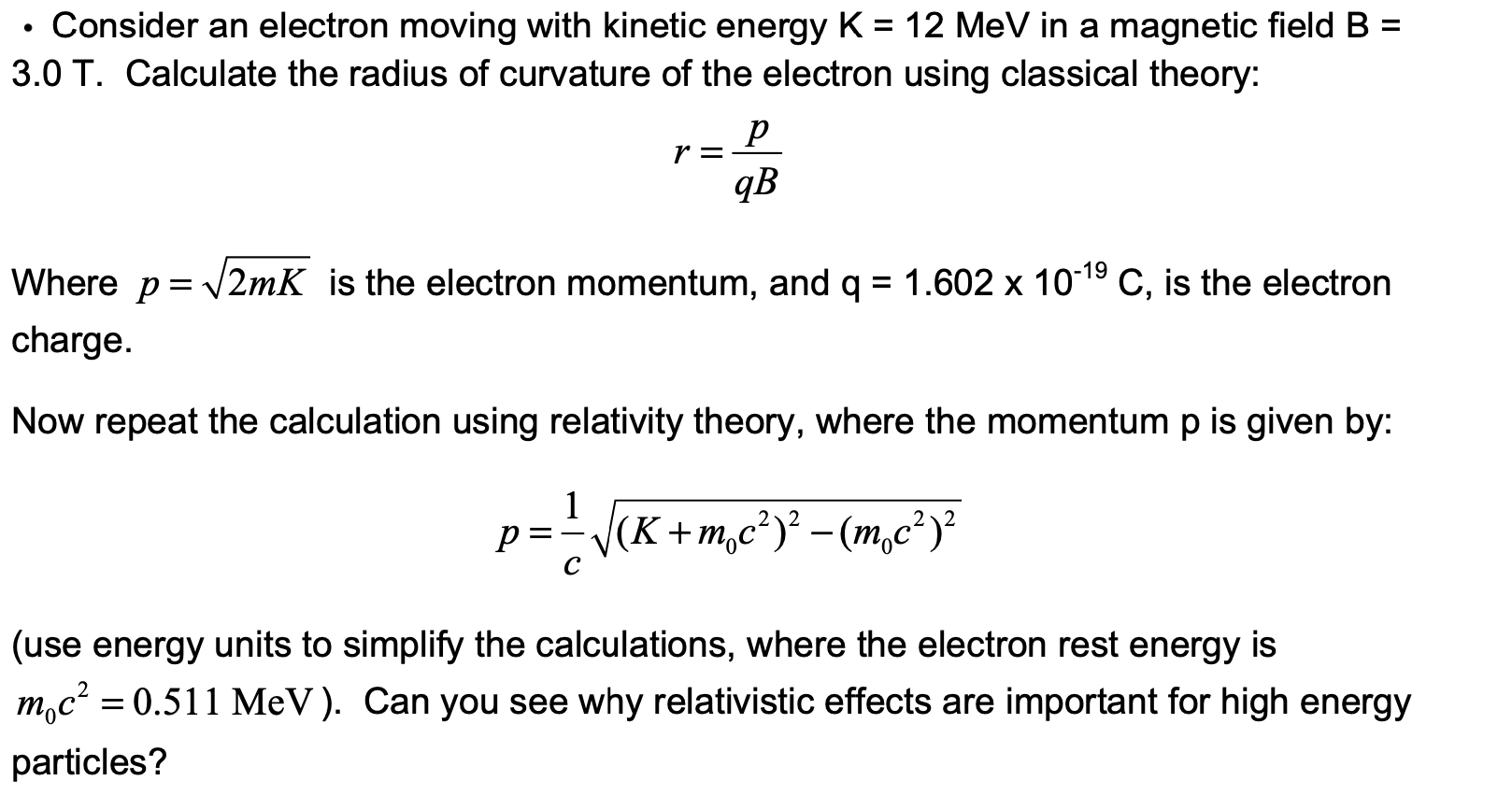
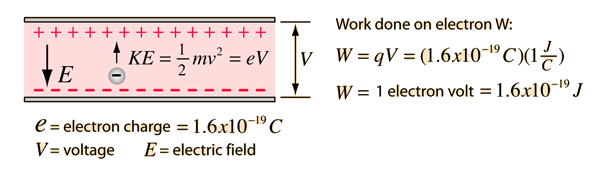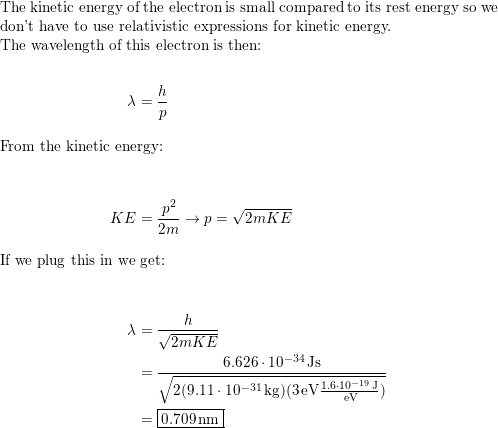The ratio of kinetic energy and total energy of an electron in a Bohr orbit of a hydrogen - like species is:
The (average) kinetic energy of the free electrons (mass = m, |charged| = e), in a metal, at a temperature T in kelvin, equals - Sarthaks eConnect | Largest Online Education Community
In the Bohr's orbit, what is the ratio of total kinetic energy and total energy of the electron - Sarthaks eConnect | Largest Online Education Community

What is the kinetic energy of an electron in electronvolts with mass equal to double its real mass? - YouTube
Using Bohr's postulates, obtain the expression for (i) kinetic energy and (ii) potential energy of the electron in stationary state of hydrogen atom. - Sarthaks eConnect | Largest Online Education Community

SOLVED: (a) What is the energy in joules and electron volts of a photon of 420- nm violet light? (b) What is the maximum kinetic energy of electrons ejected from calcium by
The kinetic energy of an electron is 5 xx 10^(5) eV (electron volts). Calculate the wavelength of the wave associated with the electron. The mass of the electron may be taken as 10^(-30) kg

homework and exercises - Is the kinetic energy of an electron always $1.6 \cdot 10^{-19}~\text{J}$? - Physics Stack Exchange
kinetic energy of an electron which is associated with de Broglie's wavelength 20 angstrom is 1)1.0eV, 2)1.51eV, 3)0.59eV, 4)0.38eV.
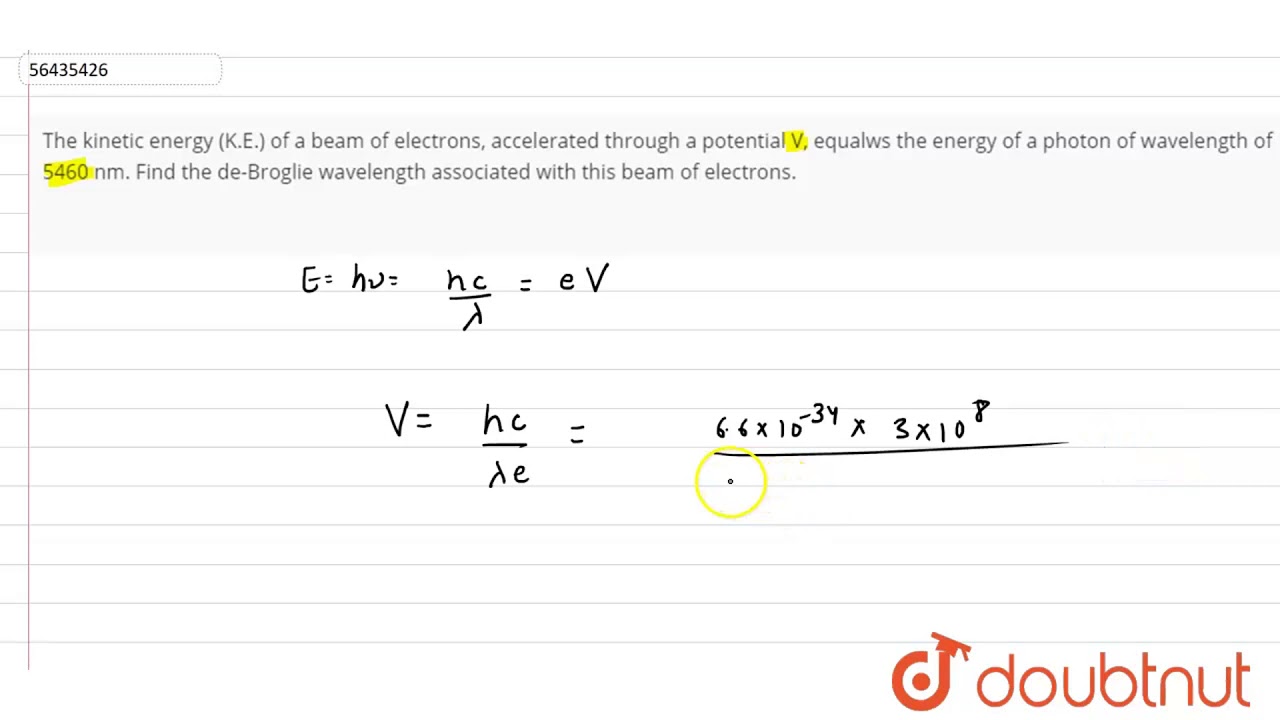





![The kinetic energy of an electron is `4.55 xx 10^(-25)J`. Calculate the wavelength . `]` - YouTube The kinetic energy of an electron is `4.55 xx 10^(-25)J`. Calculate the wavelength . `]` - YouTube](https://i.ytimg.com/vi/He9LjP05EHA/maxresdefault.jpg)