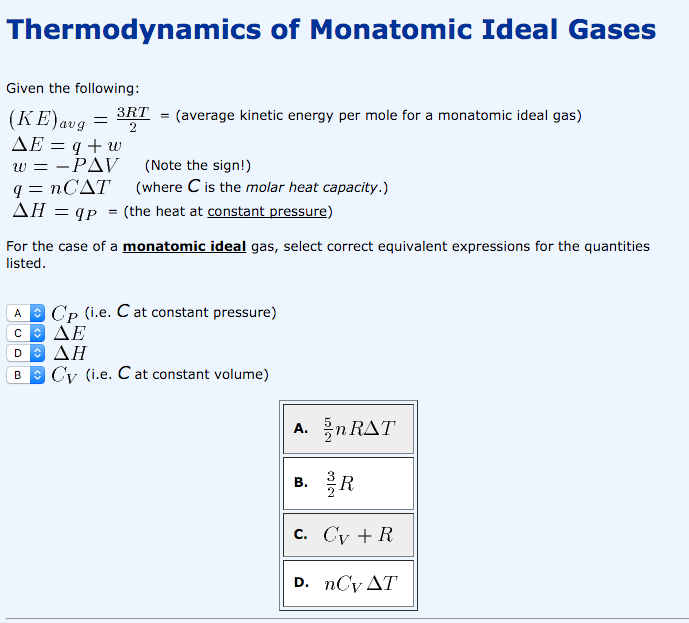
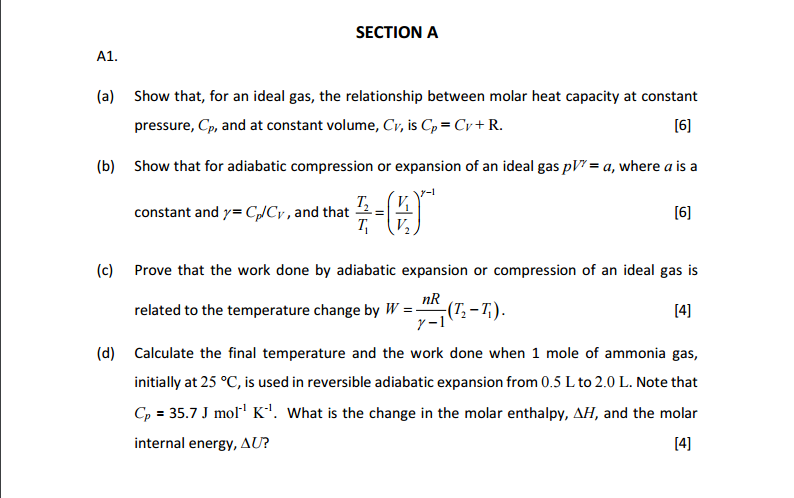
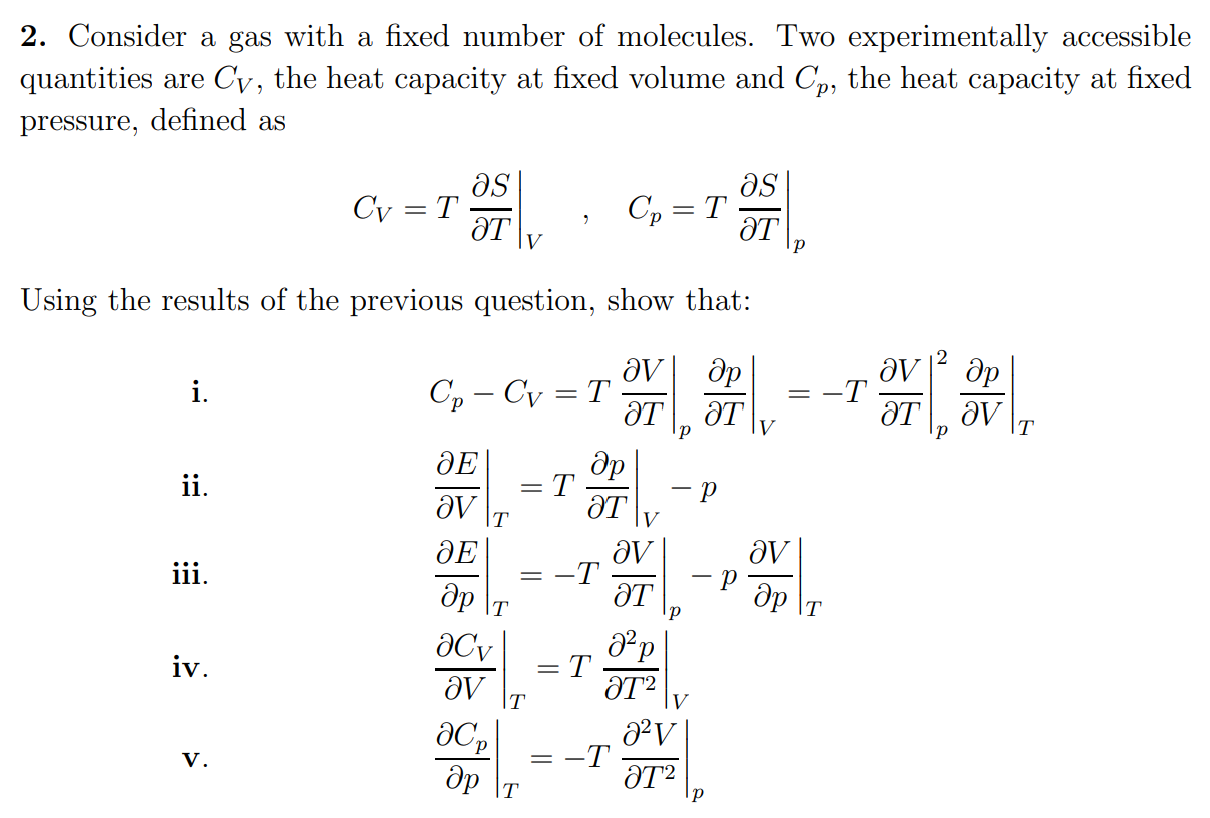
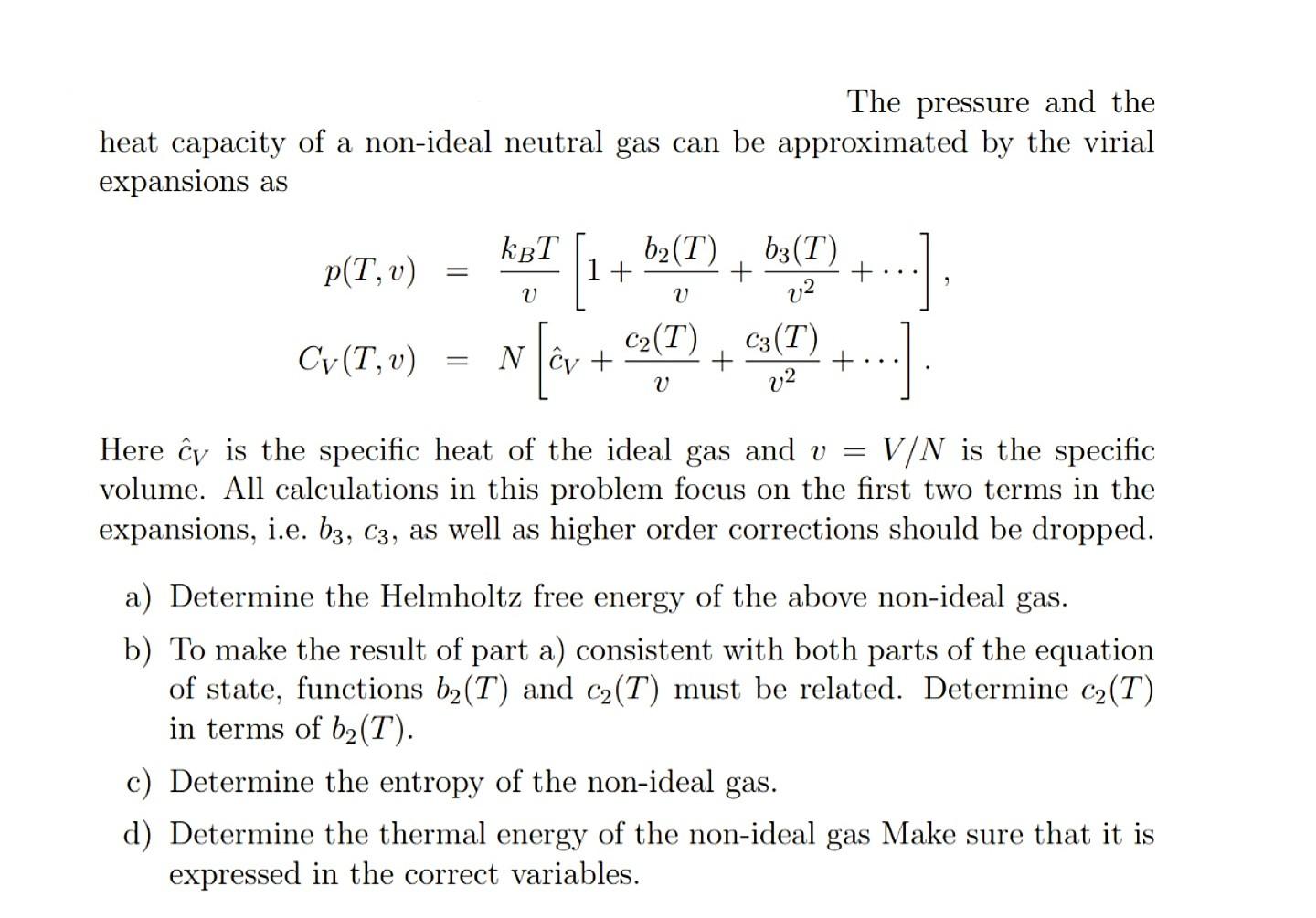
17. The molar heat capacity at constant pressure of an8Rideal gas mixture is3The ratio of molar heatcapacities at constant pressure to constant volumefor this mixture will be(1) 1.5(2) 1.4(3) 1.6(4) 1.2

Molar heat capacity of an ideal gas in the process PV^(x) = constant , is given by : C = (R)/(gamma-1) + (R)/(1-x). An ideal diatomic gas with C(V) = (5R)/(2) occupies

Find the molar heat capacity (in terms of `R`) of a monoatomic ideal gas undergoing the process - YouTube

The molar heat capacity C for an ideal gas going through a given process is given by `C=a/ - YouTube

An ideal gas undergoes a quasi static, reversible process in which its molar heat capacity C remains constant. If during this process the relation of pressure P and volume V is given

Molar heat capacity of an ideal gas varies as C = C(v) +alphaT,C=C(v)+betaV and C = C(v) + ap, where alpha,beta and a are constant. For an ideal gas in terms of

If Cp and Cv are molar specific heats of an ideal gas at constant pressure and volume respectively. If gamma is ratio of two specific heats and R is universal gas constant

thermodynamics - Derivation of heat capacity at constant pressure and temperature - Physics Stack Exchange

n-moles of an ideal gas with constant volume heat capacity CV undergo an isobaric expansion - YouTube

PHY1039 Properties of Matter Heat Capacity of Ideal Gases (C P and C V ) and Adiabatic Expansion of Ideal Gas (See Finn's Thermal Physics, Ch. 4) March. - ppt download
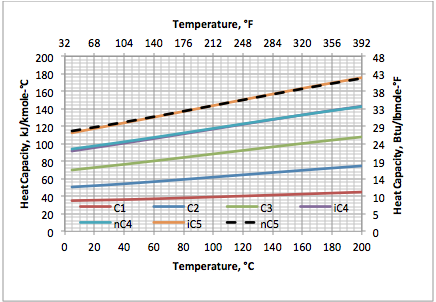
Variation of Ideal Gas Heat Capacity Ratio with Temperature and Relative Density | Campbell Tip of the Month