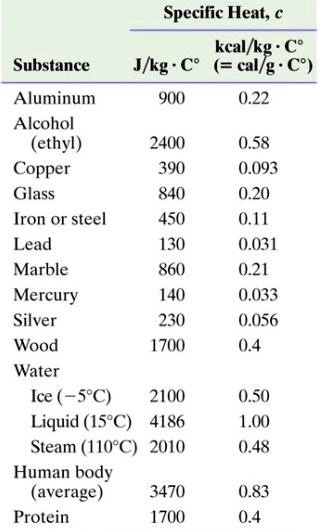
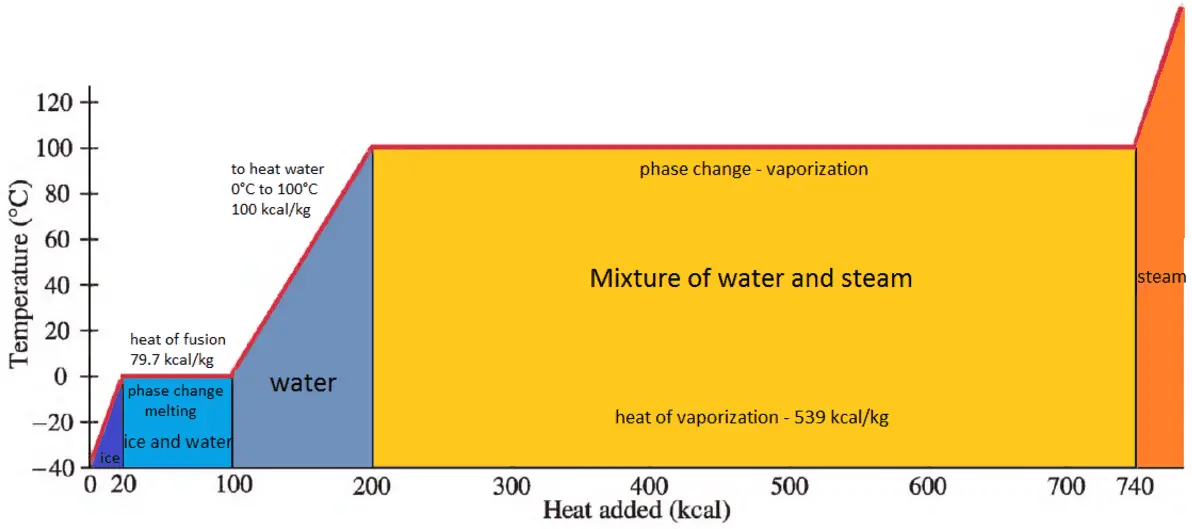
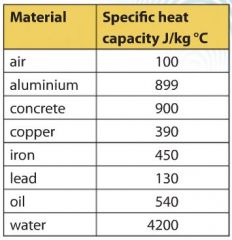
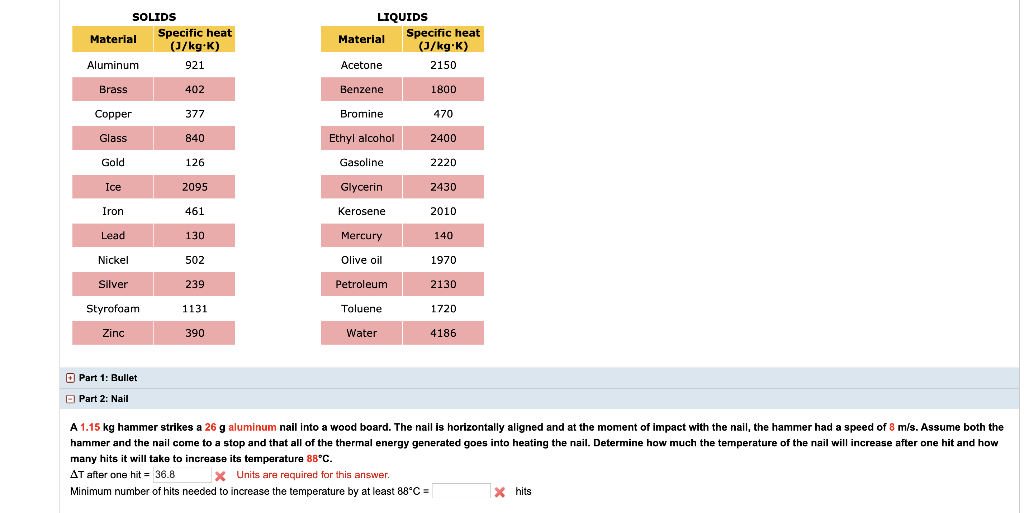
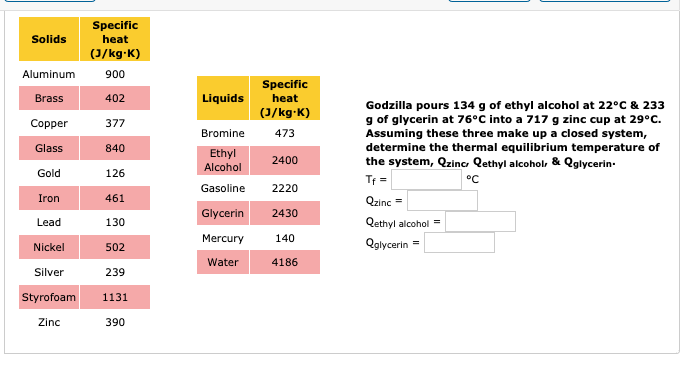
500 g of water at 100^∘C is mixed with 300 g at 30^∘C . Find the temperature of the mixture. Specific heat of water = 4.2 J g^-1 ^∘C^-1 ).
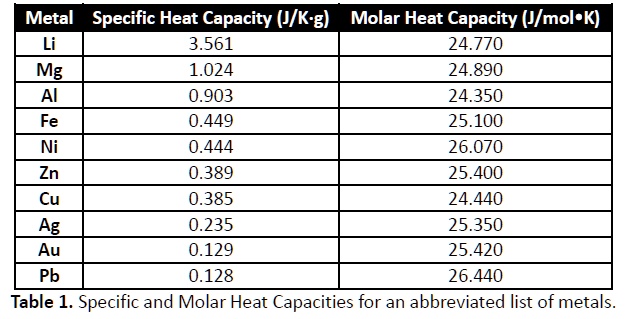
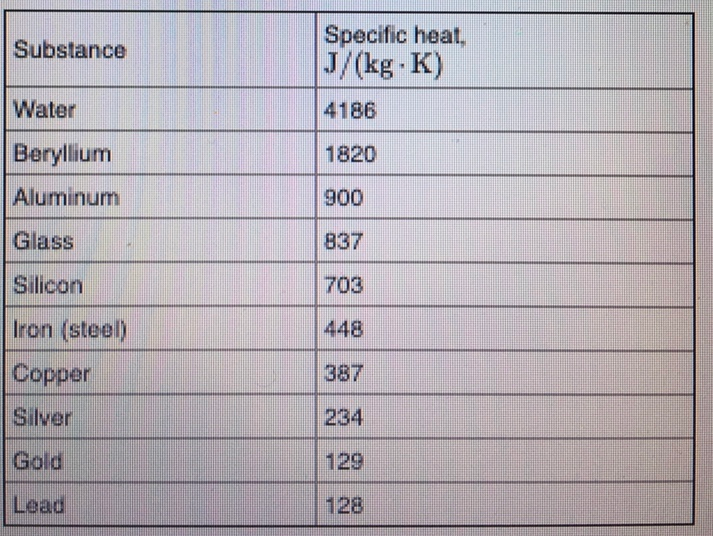
SOLVED: Metal Specific Heat Capacity (J/K-g) Molar Heat Capacity (J/mol-K) 3.561 24.770 1.024 24.890 0.903 24.350 0.449 25.100 0.444 26.070 0.389 25.400 0.385 24.440 0.235 25.350 0.129 25.420 0.128 26.440 Table 1. Specific and Molar Heat Capacities for ...
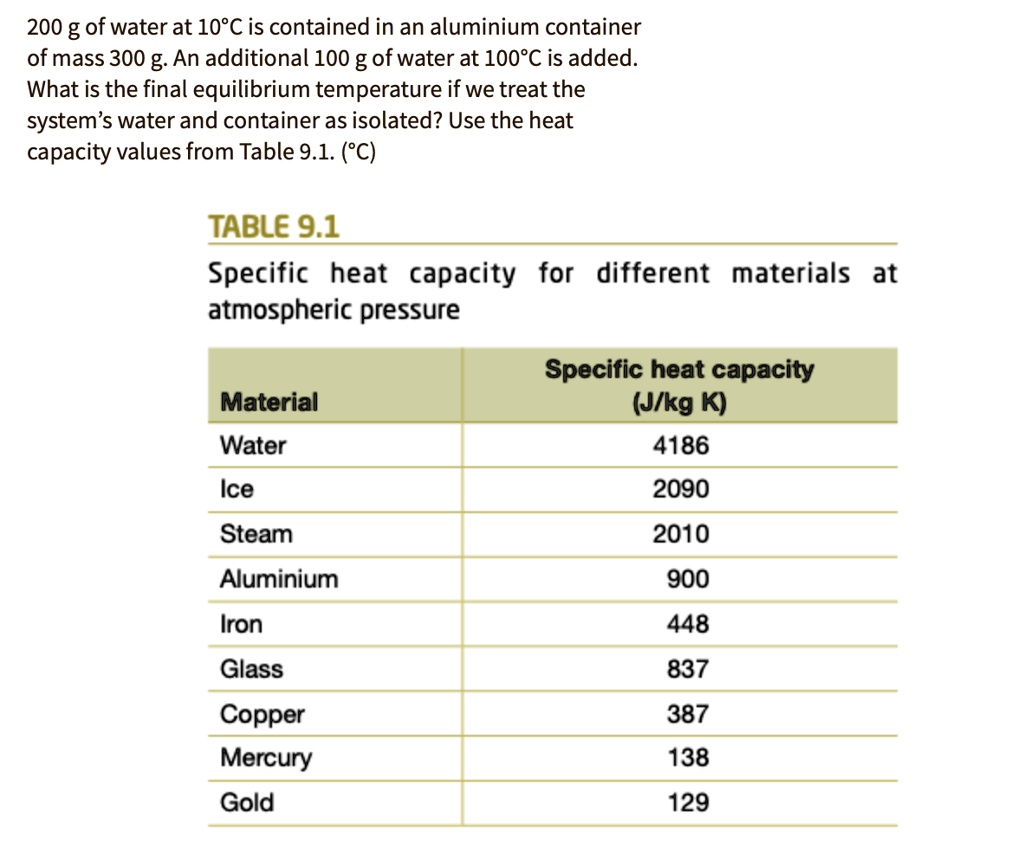
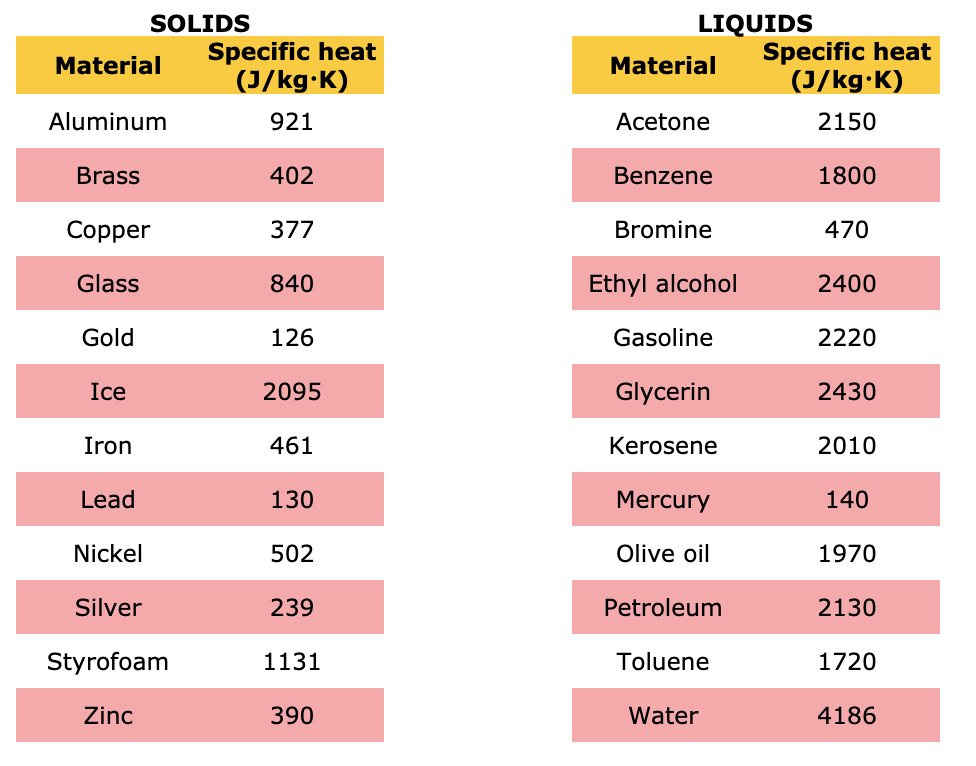
SOLVED: 200 g of water at 10*C is contained in an aluminium container of mass 300 g. An additional 100 g of water at 100*C is added: What is the final equilibrium
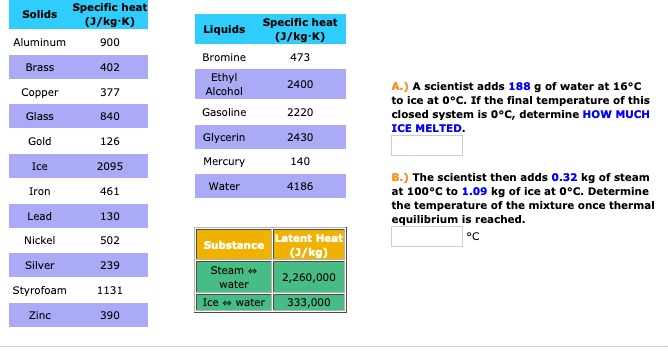
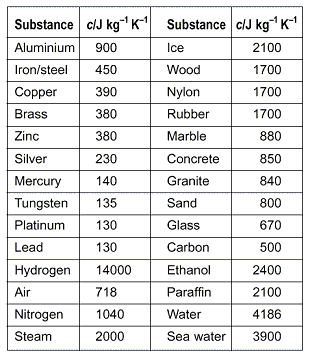
SOLVED: Specific heat Solids (J/kg K) Specific heat Liquids (J/kg K) Bromine 473 Ethyl 2400 Alcohol Aluminum 900 Brass 402 scientist adds 188 water at 16'C at 0PC: If the final temperature